Views: 0 Author: Site Editor Publish Time: 2025-11-11 Origin: Site
In the fast-paced world of modern healthcare, precise blood pressure measurement is more than a routine check—it is a cornerstone of patient safety and clinical decision-making. As clinics, hospitals, and home care services increasingly move away from traditional mercury devices, Aneroid Sphygmomanometer has emerged as a preferred solution for its accuracy, eco-friendliness, and portability. Selecting the right OEM sphygmomanometer supplier is crucial for procurement teams, distributors, and medical project contractors who require dependable, certified, and customizable devices for a variety of clinical environments. Ruian Sunnyworld International Trade Co., Ltd. and Sunnyworld Medical Instruments Co., Ltd. have established themselves as leading manufacturers in this space, offering an extensive range of professional aneroid sphygmomanometers that combine regulatory compliance, long-term durability, and flexible OEM options.
Before committing to bulk orders, it is essential that procurement teams verify that their aneroid sphygmomanometer supplier adheres to rigorous international standards. CE certification confirms compliance with European Union health, safety, and environmental requirements, signaling that the device can be legally marketed and used across Europe. ISO 13485 certification, a globally recognized quality management system for medical devices, demonstrates that the manufacturer maintains strict quality controls throughout production, from raw material sourcing to final inspection.
For U.S. markets, FDA 510(k) clearance ensures that the device is safe, effective, and substantially equivalent to previously cleared devices. Additionally, Free Sale Certificates indicate that the device meets local regulations in various regions, allowing distributors to confidently market the product internationally. Verification of these documents should include reviewing the certificate copies, checking validity dates, and confirming authenticity with relevant regulatory authorities. This process not only safeguards compliance but also reinforces the reliability of the device in clinical practice.
Regulatory compliance alone does not guarantee quality. Clinical buyers must also perform thorough checks on gauge accuracy, cuff integrity, tubing durability, and overall device safety. Clinical-grade aneroid sphygmomanometers should provide readings within ±3 mmHg, ensuring precise monitoring for both hypertension management and routine clinical assessments. Safety checks include confirming that the device components are free from defects, resistant to physical stress, and safe for repeated use. Comprehensive documentation—ranging from calibration certificates and technical manuals to safety instructions and warranty information—enables procurement teams to track quality and streamline internal audits. Ensuring these elements are in place reduces risks of errors, patient harm, and potential legal issues.
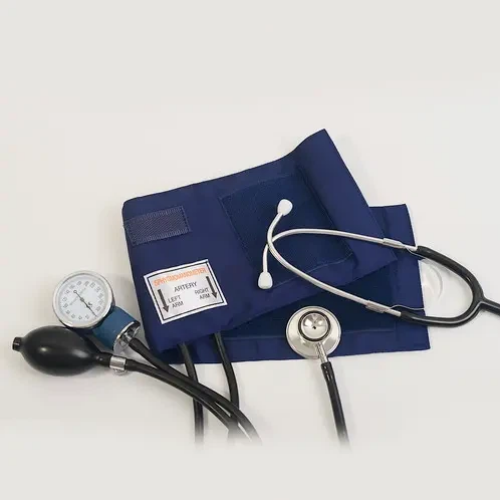
An in-depth review of technical specifications is critical when selecting an aneroid sphygmomanometer. The gauge, as the device's core measurement component, must retain accuracy over long-term use. This includes evaluating mechanical robustness, dial clarity, and needle stability. Casing materials are equally important; premium materials protect internal components from shocks, moisture, and wear.
Cuff range is another vital consideration. Clinics should maintain multiple sizes, including adult, pediatric, large, and neonatal options, to ensure accurate readings across patient demographics. Proper cuff fit minimizes measurement errors that could affect diagnosis and treatment. High-quality cuffs are made with durable fabrics, leak-resistant tubing, and reliable Velcro closures.
Packaging standards influence the device's condition upon delivery. Devices should be securely packed in protective materials that prevent damage during shipping and storage. Suppliers like Sunnyworld Medical adhere to stringent packaging requirements to ensure devices arrive ready for immediate use, safeguarding both functionality and hygiene standards.
Modern clinical environments often require flexibility. Portable aneroid sphygmomanometers are advantageous for mobile health services, home visits, and field clinics. Lightweight designs with compact gauges and flexible tubing reduce strain during repeated use and enhance workflow efficiency. Ergonomic considerations—such as comfortable hand bulbs, easy-to-read dials, and adjustable cuffs—further support healthcare professionals in providing accurate and stress-free measurements. Additionally, certain models include anti-slip bases and reinforced tubing, which improve reliability in high-traffic clinic settings.
Negotiating commercial terms is an essential part of the procurement process. Minimum order quantities (MOQs) impact inventory management and budgeting. Reliable OEM suppliers provide clear policies, allowing buyers to align orders with demand without overstocking.
Lead times should be explicitly discussed to ensure timely delivery for project rollouts or seasonal demand spikes. Reputable suppliers also offer sample policies, enabling buyers to inspect, test, and validate device quality before placing bulk orders. This step is critical for maintaining confidence in device performance and ensuring that the purchased units meet clinical requirements.
OEM customization enhances branding and usability. Typical options include selecting device color, applying custom logos, and translating instruction manuals into local languages. Clinics and distributors can personalize devices to match organizational identity or local regulations while ensuring that functionality and compliance are maintained. Sunnyworld Medical supports these options with flexible, scalable solutions suitable for both small and large-scale projects. Custom packaging and bundled kits for multiple cuff sizes can also be arranged to simplify logistics for distributors.
Post-purchase support is crucial for maintaining device accuracy and longevity. Annual calibration ensures continued precision, while routine preventive checks prevent premature wear. Suppliers provide guidance on establishing local service arrangements or remote support, making it easier for clinics to maintain devices without relying on external contractors.
Warranty coverage protects buyers against defects and component failures. High-quality OEM providers supply readily available replacement parts, including cuffs, bulbs, and valves, enabling uninterrupted clinical operations. Effective spare parts management reduces downtime, ensures consistent device performance, and supports long-term trust in the supplier. Many manufacturers also provide online portals with instructional videos and downloadable guides to help local staff handle basic preventive maintenance safely and efficiently.
Mitigating procurement risks involves detailed evaluation of potential suppliers. Requesting quality control (QC) reports, batch testing results, and production audits helps confirm consistency and reliability. Factory inspections, either virtual or on-site, provide insight into manufacturing processes, hygiene standards, and adherence to ISO and CE regulations. Sampling devices from different production batches further ensures that all units meet specified performance criteria. Thorough evaluation minimizes operational risks and enhances confidence in device accuracy and durability. Procurement teams should also consider supply chain traceability, ensuring components are sourced from reputable vendors and meet safety standards.
Aneroid sphygmomanometers are not only used in traditional hospital settings but are increasingly deployed in outpatient clinics, mobile health units, home healthcare, and telemedicine services. Accurate, portable devices allow clinicians to perform vital blood pressure checks in field conditions, remote areas, or during patient home visits without sacrificing precision. Integration with existing patient monitoring protocols is seamless, particularly when devices come with standardized color-coded cuffs and clear operational instructions. Proper training for staff ensures correct usage, reducing human errors and maximizing device reliability.
Choosing a reliable Aneroid Sphygmomanometer supplier requires balancing regulatory compliance, technical specifications, commercial terms, after-sales support, risk management, and practical clinical applications. By systematically verifying certifications, assessing product quality, negotiating OEM options, and establishing robust support systems, procurement teams and distributors can ensure accurate, durable, and safe blood pressure monitoring solutions. Ruian Sunnyworld International Trade Co., Ltd. and Sunnyworld Medical Instruments Co., Ltd. deliver high-quality, mercury-free devices designed for diverse clinical environments, combined with professional technical support and customizable OEM solutions. For personalized quotations, factory audit packs, and sample orders, contact us today to start your procurement process.
1. How do I ensure proper cuff selection for diverse patient populations?
Evaluate arm circumference carefully and choose from adult, pediatric, large, and neonatal cuffs. Correct sizing ensures precise readings and minimizes measurement errors.
2. What additional features enhance usability in busy clinical environments?
Features such as anti-slip bases, reinforced tubing, ergonomic bulbs, and clear gauge markings improve comfort, efficiency, and measurement reliability.
3. Can mobile healthcare teams rely on aneroid sphygmomanometers for fieldwork?
Yes. Lightweight, portable, and durable models maintain accuracy in various environments, including home visits, outreach programs, and temporary clinics.
4. How do suppliers support multi-region distribution and compliance?
Professional OEM suppliers provide translated manuals, Free Sale Certificates, and flexible packaging solutions to meet regional regulatory requirements and ease logistics.
